WHO rejects Presidential Spoeksperson's claims
The first operation of the revamped President's Media Division is a failure
The World Health Organisation (WHO) has denied claims that President Gotabaya Rajapaksa had influenced the decision by the WHO to approve the emergency use of the Sinopharm vaccine, the Daily Mirror reported.
President's Spokesman Kingsley Rathnayaka on Monday (10) said that that the WHO had approved the Sinopharm vaccine as a result of a discussion between President Gotabaya Rajapaksa and WHO Director-General Dr. Tedros Adhanom Ghebreyesus.
In response to a question raised by Daily Mirror, the WHO said that the Emergency Use Listing (EUL) of the WHO process usually takes two to three months to complete, depending on the quality and availability of the data submitted by the vaccine developers, among other factors and the prequalification process is more complex and thus takes longer.
“Domestic emergency use authorizations are issued at the discretion of countries and not subject to WHO approval,” the WHO said in an emailed response to Daily Mirror.
However, the statement made by President's Spokesperson Kingsley Rathnayaka stated that;
"Meanwhile, Sri Lanka received a donation of 600,000 doses of Chinese Sinopharm vaccine, which was not earlier approved by the World Health Organization.
However, as a result of a discussion President Gotabaya Rajapaksa held with the Director-General of the World Health Organization (WHO) via ‘Zoom’ last Friday, the WHO on the same evening approved the emergency use of the “Sinopharm ” vaccine.
Accordingly, the “Sinopharm” vaccination programme was commenced from the very next day of receiving approval and the government is making arrangements to vaccinate 25,000 people per day in the Western Province under the first phase.
Negotiations held with the Chinese authorities to obtain 3 million doses of the Sinopharm vaccine have been successful and the government has made plans to commence the vaccination programmes in other provinces too as soon as the vaccines are received."
What do we know about the Chinese vaccines?
According to Sri Lanka Brief, the decision to approve the Sinopharm vaccine was taken by WHO’s technical advisory group, which met since April 26 to review the latest clinical data and manufacturing practices.
It was reported that the WHO is likely to approve the Sinopharm vaccine by the end of this week if the Chinese government provides the necessary information, adding that the Russian Sputnik vaccine will not be approved until next July.
Therefore, it is quite clear that the Sinopharm vaccine was likely to be approved very soon.
At the same time, if he was responsible for getting WHO approval for the Sinopharm vaccine as his spokesperson claimed, why were the leaders of countries like Turkey, Hungary and Indonesia, which also uses the Sinopharm vaccine, not able to get approval earlier?
President Rajapaksa spoke with WHO chief Dr Tedros Adhanom Ghebreyesus, on Friday. By then however, the technical advisory group had already decided to approve the Sinopharm vaccine for emergency use. Accordingly, 'Sri Lanka Brief' noted yesteday (10) that President Rajapaksa's claim that he was instruemntal in getting WHO approval for the Sinopharm vaccine was 'bogus'.
According to Sri Lanka Brief, the WHO on May 04 publicised a report on the vaccine evaluation process which clearly showed that the evaluation of the Sinopharm was to be finalised by early May.
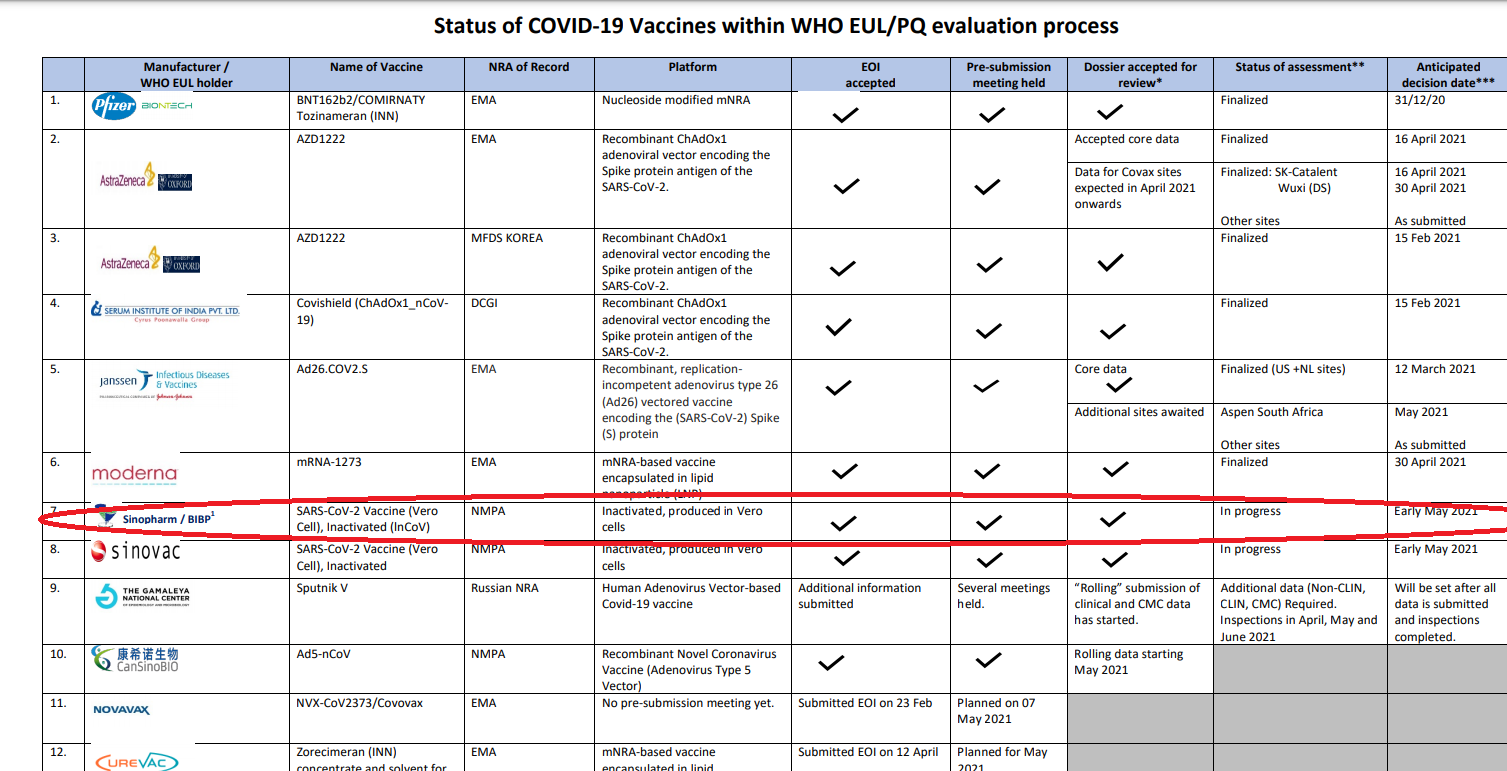 Source: Sri Lanka Brief
Source: Sri Lanka Brief
"If Sri Lanka President Rajapaksa’s claim is right, that will be another questionable issue of WHO’s giving in to Chinese pressure," Sri Lanka Brief pointed out.